HYDRONICS WORKSHOP
BY JOHN SIEGENTHALER
What’s the difference?
Using antifreeze vs. water in hydronic circuits.

At times, I’ve been known to throw out a rather “snide” comment when the subject turns to using antifreeze in hydronic systems:
“The only good thing about antifreeze is that it doesn’t freeze.”
This quip was based on the facts that using antifreeze in a hydronic system:
- A. Adds to the cost of the system.
- B. Increases the pumping power required to achieve the given rate of heat transfer, relative to water.
- C. Decreases the heat storage ability of the system fluid, relative to water.
- D. Needs to be periodically checked for chemical stability.
Still, when it comes to worst case conditions (e.g., subfreezing air temperatures for hours or days, no electric power, and no one available to open drain valves in the system, an antifreeze solution in the system is the only way to prevent freezing.
Ultimate Resiliency: One concept that’s getting increasing attention among HVAC engineers is “resilient design.” It refers to approaches that - ideally - allow systems to remain operational under very abnormal conditions, or minimize effects that could cause major damage to systems when and if they are otherwise rendered inoperative.
The methods used by different designers to achieve what they consider resilient design vary. For example: One designer might think that including an automatic-start backup generator for a building with the hydronic system provides sufficient protection against freezing during a utility power outage. It would, as long as the generator starts when it’s supposed to and has enough fuel to keep running for the duration of the outage. Another, more conservative, designer may think that the risk of the generator not starting, or that the power outage might outlast the generator’s fuel supply, fails to meet their standard of resilient design. It’s likely that the latter designer would specify antifreeze in the entire hydronic system to ensure that freezing would not occur, regardless of the duration of the outage.
Different Brews: Over the last several decades, there have been several fluids that have been mixed with water to produce antifreeze solutions. They include inhibited ethylene glycol, inhibited propylene glycol, methanol, ethanol, calcium chloride and other salts.
All these chemistries have pros and cons. For example, methanol solutions, which have had some acceptance in the geothermal heat pump industry, have lower viscosity and better specific heat than equivalent glycol solutions. But, methanol is both toxic and highly flammable. Ethanol (the alcohol used in “booze”) is a controlled chemical. It typically requires a license to purchase. There are also government restrictions on how these flammable materials can be legally transported. You can’t just put a 55 gallon drum of methanol in your pickup, throw on a couple of straps, and legally head down the highway. Not convinced? Imagine an installer taking their lunch break while sitting on a 5-gallon pail filled with methanol, with the cap off. He accidentally drops his cigarette on the pail. You take it from there…
Salts such as calcium chloride can depress the freezing point of water, but their “Achilles Heel” is corrosion, especially on ferrous metals.
The most commonly used antifreeze fluids used in HVAC systems are ethylene glycol and propylene glycol. Ethylene glycol, although having more desirable hydraulic and thermal properties compared to propylene glycol, is toxic in relatively small amounts. In my opinion, that has the potential to create serious legal liability in residential HVAC applications, especially considering the “warped reality” depictions created by clever trial attorneys, and the hyper-empathetic verdicts delivered by some juries.
My preference for antifreeze is inhibited propylene glycol. I would wager that everyone reading this article has, at times, ingested some amount of “food grade” propylene glycol. It’s used in eye drops, candy, soft drink, acetaminophen, aspirin and the list goes on.
However, “food grade” propylene glycol is not an ideal antifreeze fluid for hydronic systems. It can degrade at elevated temperatures, support microbes, and eventually become acidic with age and elevated temperatures. That’s where the word “inhibited” comes in. Inhibited propylene glycol contains chemical additives to better stabilize the fluid, and reduce corrosion potential between the fluid and specific metals in the system (copper, iron, aluminum etc.).
Thermal Considerations: The rate at which any single-phase fluid conveys thermal energy can be determined by formula 1.
Formula 1:

Where:
q = rate of heat transport (Btu/hr)
D = density of the fluid (lb/ft3), which depends on the fluid’s temperature.
c = specific heat of the fluid (Btu/lb/ºF), which also depends on the fluid’s temperature.
f = flow rate of fluid (US gallons per minute)
∆T = temperature change of the fluid as it absorbs or releases heat though some device. (ºF)
8.01 = a constant needed to make the units work out correctly.
The higher the fluid’s density, and the higher its specific heat, the greater the rate of heat transfer for a given flow rate and given temperature change.
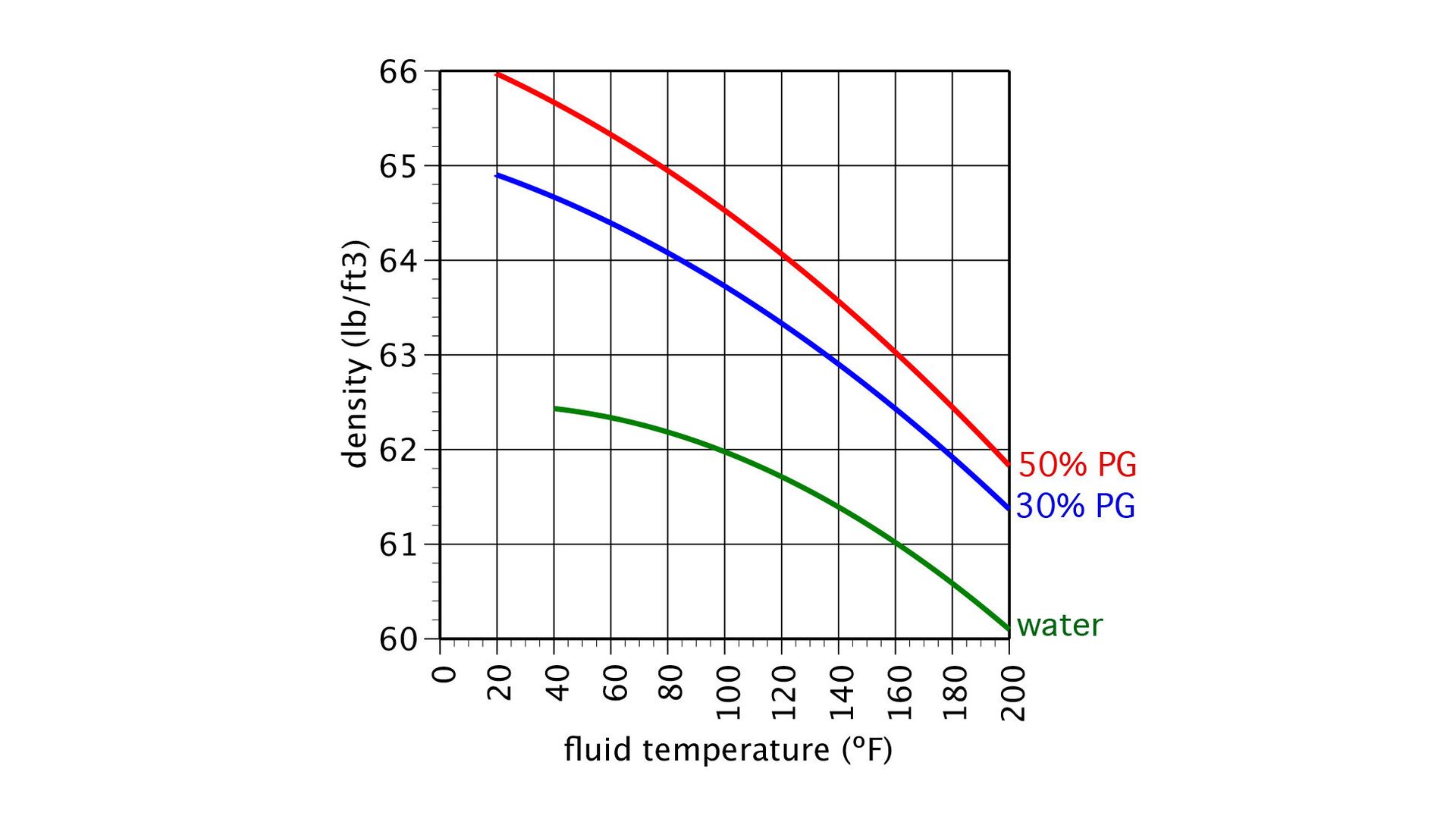
Figure 1 shows the density of water, along with that of 30% and 50% solutions of one commercially available inhibited propylene glycol.
ENLARGE
FIGURE 1
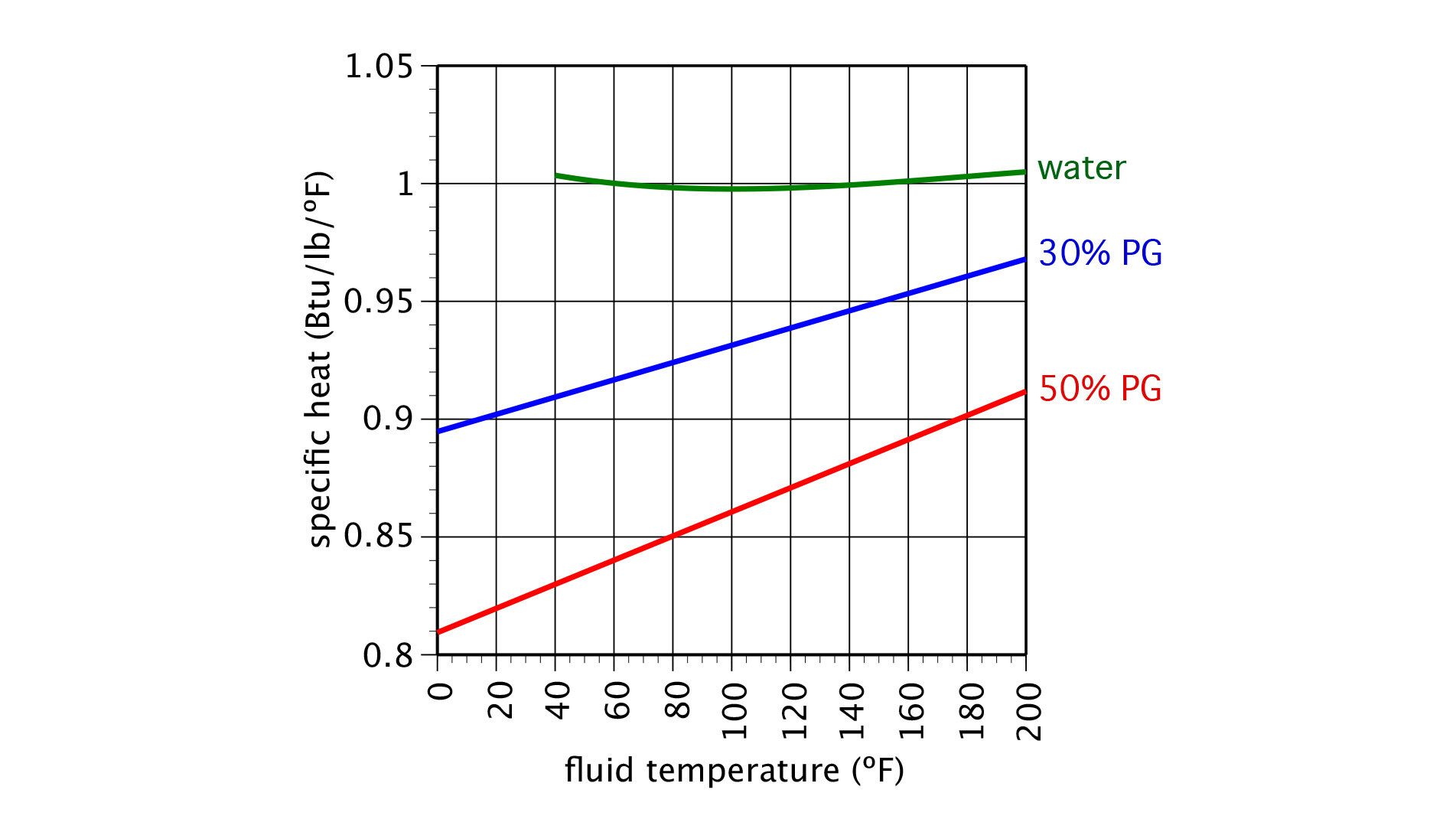
Figure 2 shows the specific heat of these same fluids.
ENLARGE
FIGURE 2
The density of all the fluids decreases with increasing temperature, while the specific heat of the two glycol solutions increases with increasing temperature. The specific heat of water varies insignificantly over the temperature range shown in figure 2.
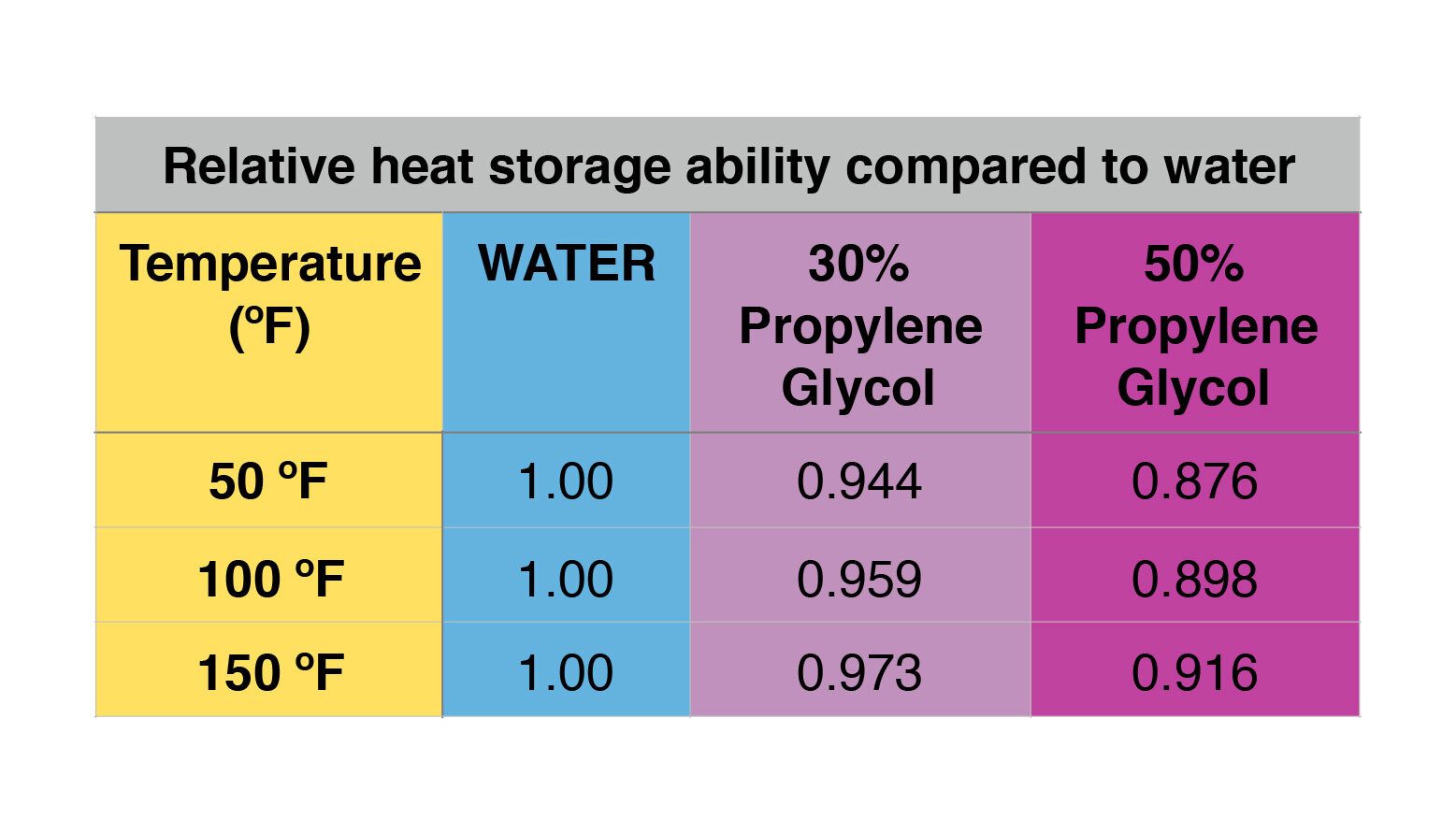
The multiplication of density (D) times specific heat (c) represents the “net effect” of how different fluids effect heat transfer. Figure 3 shows a relative comparison of this multiplication at three different temperatures 50,100, and 150 ºF. In each case, the multiplication (D x c) for the glycol solutions is given as a percentage of that for water. This allows for a comparison of the heat storage ability of antifreeze, relative to that of water.
ENLARGE
FIGURE 3
These numbers show that the heat storage ability of glycol solutions is not as good as that of water. This is especially true at lower fluid temperatures and higher glycol concentrations.
Still, at reasonably “hot” temperatures such as 150 ºF, the heat storage ability of a 30% solution of propylene glycol is only about 3% lower than that of water. This would imply that a storage tank would need to hold about 3% more 30% PG solution compared to water, to store the same amount of heat, and while undergoing the same temperature change.
It’s a Drag: Another important characteristic of glycol solutions is that they have higher viscosity than water at the same temperature. This property, in combination with higher density compared to water results in more head loss (and associated pressure drop) through any hydronic circuit, and at any given flow rate. Higher head loss implies that more pumping power is needed to achieve and maintain a given flow rate through a given circuit compared to operating the same circuit with water.
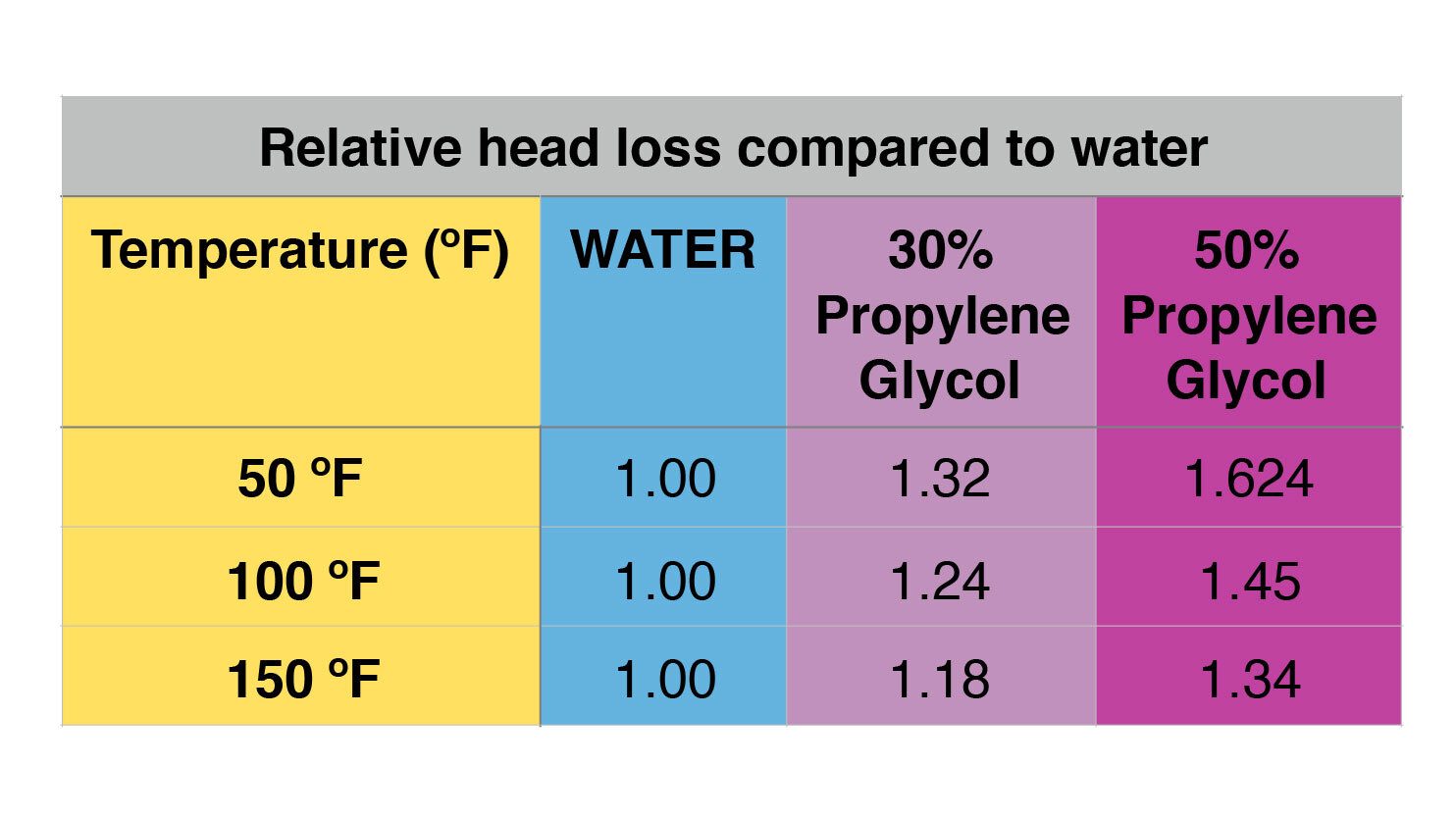
The table in figure 4 shows another relative comparison of the head loss of 30% and 50% solutions of propylene glycol at the same three temperatures used in figure 3.
Figure 4:
ENLARGE
FIGURE 4
For example, the head loss of a 30% solution of propylene glycol at 100 ºF is 1.24 times higher than for water at 100 ºF, in the same circuit, and at the same flow rate.
The head loss of a high percentage glycol solution relative to water is very significant at lower fluid temperatures. A good example is the use of a 30% solution of propylene glycol in an earth loop for a geothermal heat pump system. At a fluid temperature of 32ºF, the head loss of a 30% PG solution is approximately 40% higher than that caused by water. Some earth loops can operate at temperatures down to around 25 ºF, which would push viscosity and resulting head loss even higher. This must be taken into account when sizing earth loop circulator(s).
The combined effects of lower specific heat and higher viscosity imply both higher flow rates and higher head loss to create the same heat transfer ability of a given circuit operating with an antifreeze solution, compared to that circuit operating with water. The combined effects are easily evaluated using software such as the Hydronics Design Studio.
To demonstrate the “net effect” of using propylene glycol solutions, I created a simple circuit consisting of 100 ft of residential fin-tube baseboard, a total circuit equivalent length of 200 feet of 3/4” type M copper, a Taco 007 circulator and a supply fluid temperature of 160 ºF. Figure 5 shows the simple circuit.
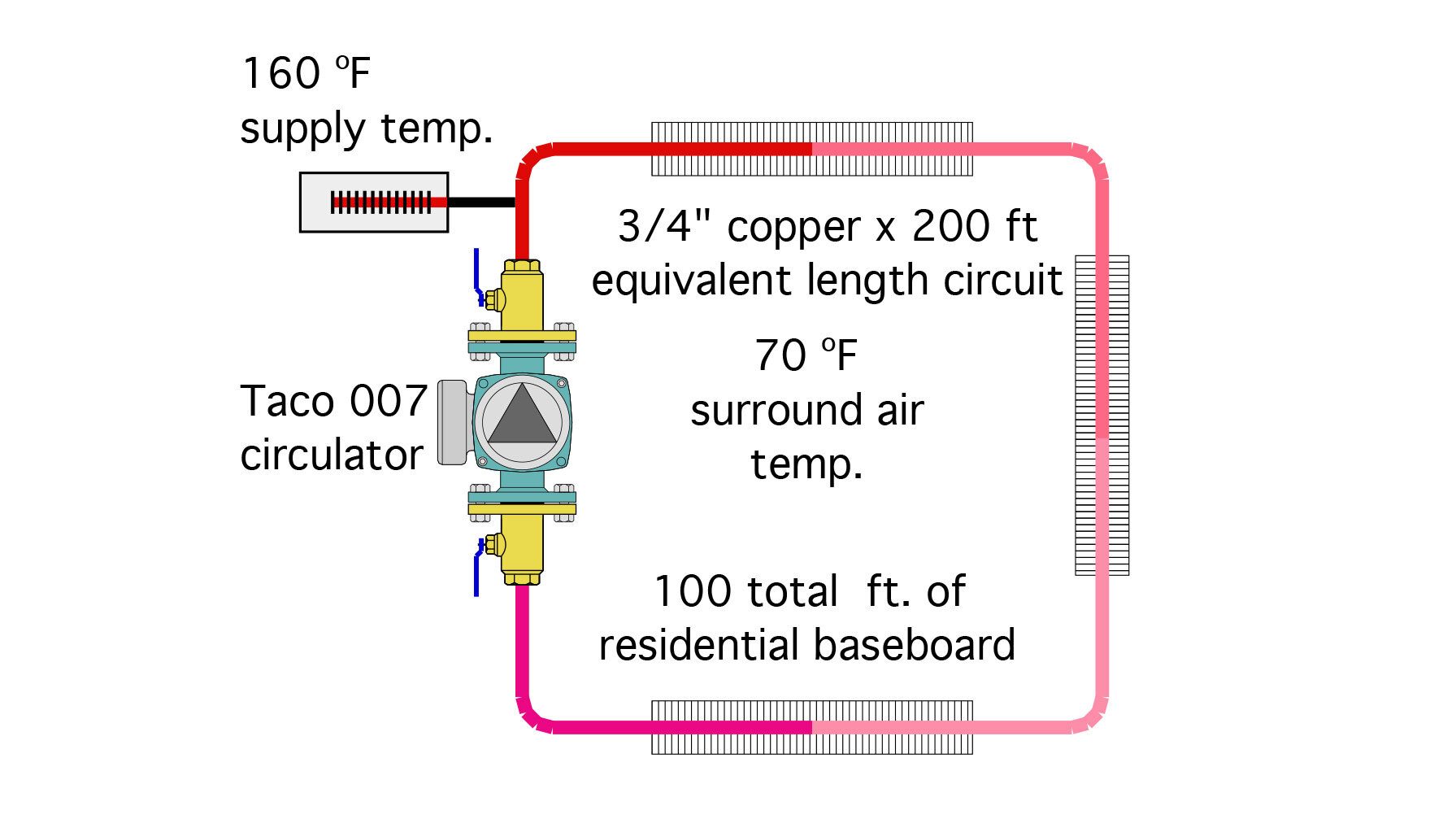
ENLARGE
FIGURE 5
Figure 6 shows the flow rate and heat output of the circuit when the fluid is changed, with all other operating conditions remaining identical. The red numbers in parentheses indicate the percentage change in flow and heat output relative to using water in the circuit.
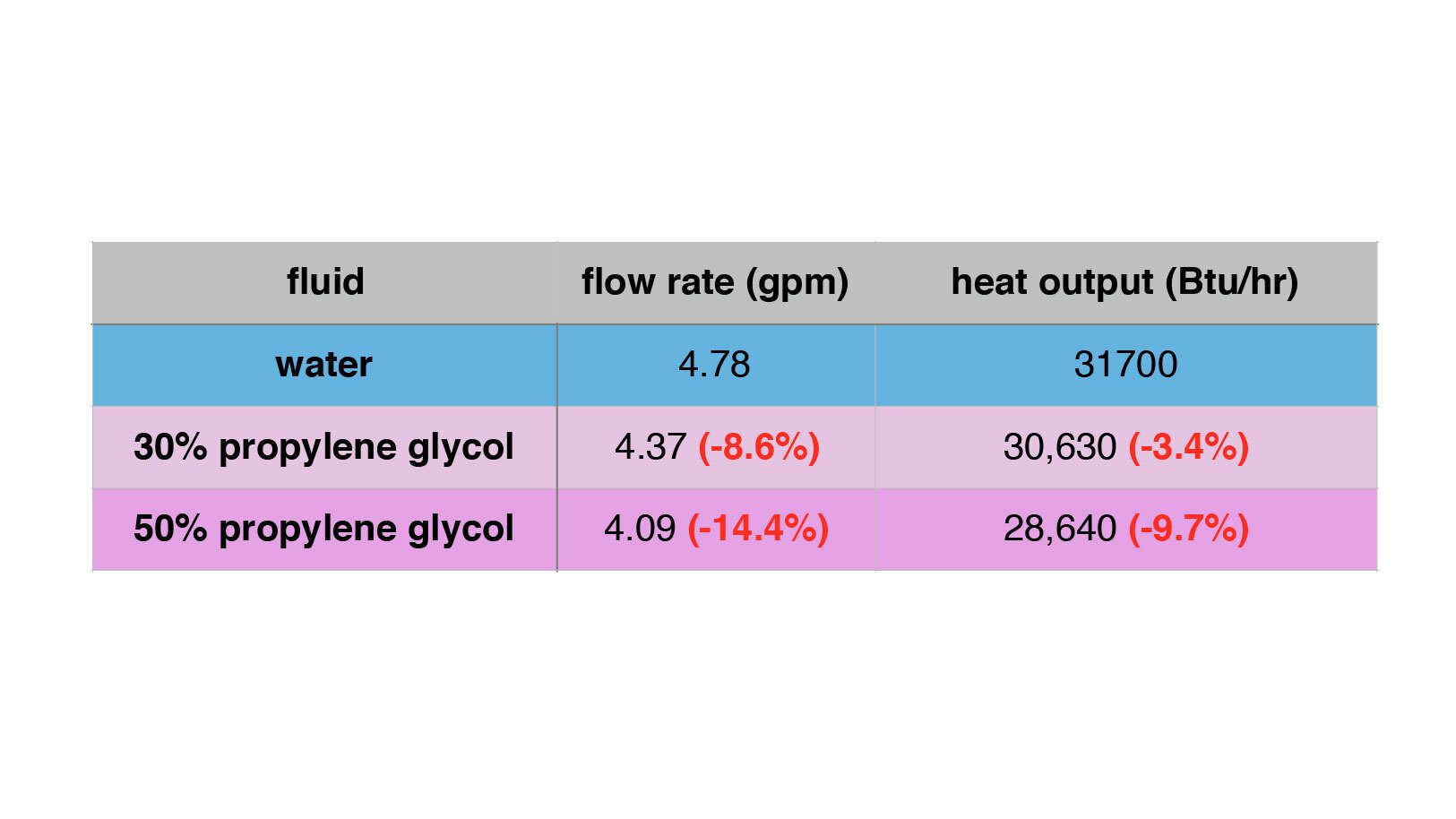
ENLARGE
FIGURE 6
The drop in heat transport rate using a 30% PG solution isn’t too bad - down just over 3%. However, the drop in heat transport rate using a 50% solution is almost 10%. That’s certainly significant.
That loss of heat transport capacity could be compensated for by 1) using a more powerful circulator or a higher speed on a multi-speed circulator, 2) increasing pipe size to reduce head loss, 3) looking for any component substitutions that could reduce head loss.
Expanded Thinking: The changes in density of propylene glycol solutions with temperature is greater than that of water. This means that expansion tanks used in systems with glycol solutions need to be larger in order to operate with the same pressure limits.
I used the Expansion Tank Sizer module in the Hydronics Design Studio software to calculate the minimum expansion tank volume for a system containing 50 gallons of fluid, with a temperature cycling range from 60ºF at cold fill, to 180 ºF at maximum temperature. The top of the system was assumed to be 10 feet above the expansion tank connection, and a 30psi relief valve was assumed. The calculated minimum expansion tank (shell) volumes for this scenario are listed in figure 7.

ENLARGE
FIGURE 7
In this case, the expansion tank volume requirement for a 30% solution of PG is about 25% greater than that required if the system operated with water.
I support the idea that reasonably oversized expansion tanks are useful in hydronic systems, especially those operating with antifreeze. One benefit of oversizing is that the tank can store a “reserve volume" of fluid that can flow into the system as air is captured and eliminated following initial start-up.
In a typical space heating system, the temperature of the fluid is unlikely to drop below that at which the system is filled and commissioned. However, in systems such as geothermal earth loops, solar thermal systems and snowmelt systems, the fluid temperature will often drop below that at which the system is filled and commissioned. In such situations it’s undesirable for the diaphragm inside the expansion tank to be fully expanded against the tank shell. If this occurs, there’s no way the expansion tank can releases more fluid back into the system as the temperature continues to drop and the fluid continues to “shrink.” This might lead to some locations in the system dropping below atmospheric pressure. Devices such as automatic air vents or air separators at such locations will act as “vacuum breakers,” allowing air into the system.
Although there are ways to calculate the extra fluid volume needed to avoid this situation, oversizing the expansion tank by 20-25%, and pumping up the system pressure an extra 5psi at commissioning is generally sufficient to ensure that some residual fluid volume is available when temperatures drop below the cold file temperature.
Water Quality: Glycol-based antifreeze should not simply be mixed with whatever water is available on the site. Ideally, they should be combined with demineralized water to minimize any chemical interaction with chlorine, minerals, microbes or other contaminants in the site water. Several companies now offer equipment to created demineralized water on site.
Don’t assume that this mixture can then be pumped into a newly-constructed system or an existing system that has been drained. New systems often contain residuals amounts of soldering flux, oils from manufacturing processes or other debris. Existing systems may contain iron oxides or residual amounts of older antifreeze solutions. Best practice is to use a “hydronic detergent,” which are available from several suppliers in North America, to internally wash the system prior to adding the antifreeze solution. Doing so will prolong the service life of the antifreeze and reduce the possibly of chemical interactions.
Concentration: Companies that supply glycol-based antifreeze often list two temperatures that apply to a given percentage concentration of their antifreeze.
The “freeze point temperature” is the lowest temperature that prevents the formation of ice crystals in the solution. The solution can operate at or above the freeze point temperature, indefinitely, without ice crystal formation.
The “burst point temperatures” is the lowest temperature at which expansion of the solution as it changes from liquid to solid will not cause pipes or other components to fracture. The burst point temperature is significantly lower than the freeze point temperature.
Figure 8 shows how the freeze point temperature and burst point temperature of one commercially available propylene-glycol antifreeze vary with temperature.
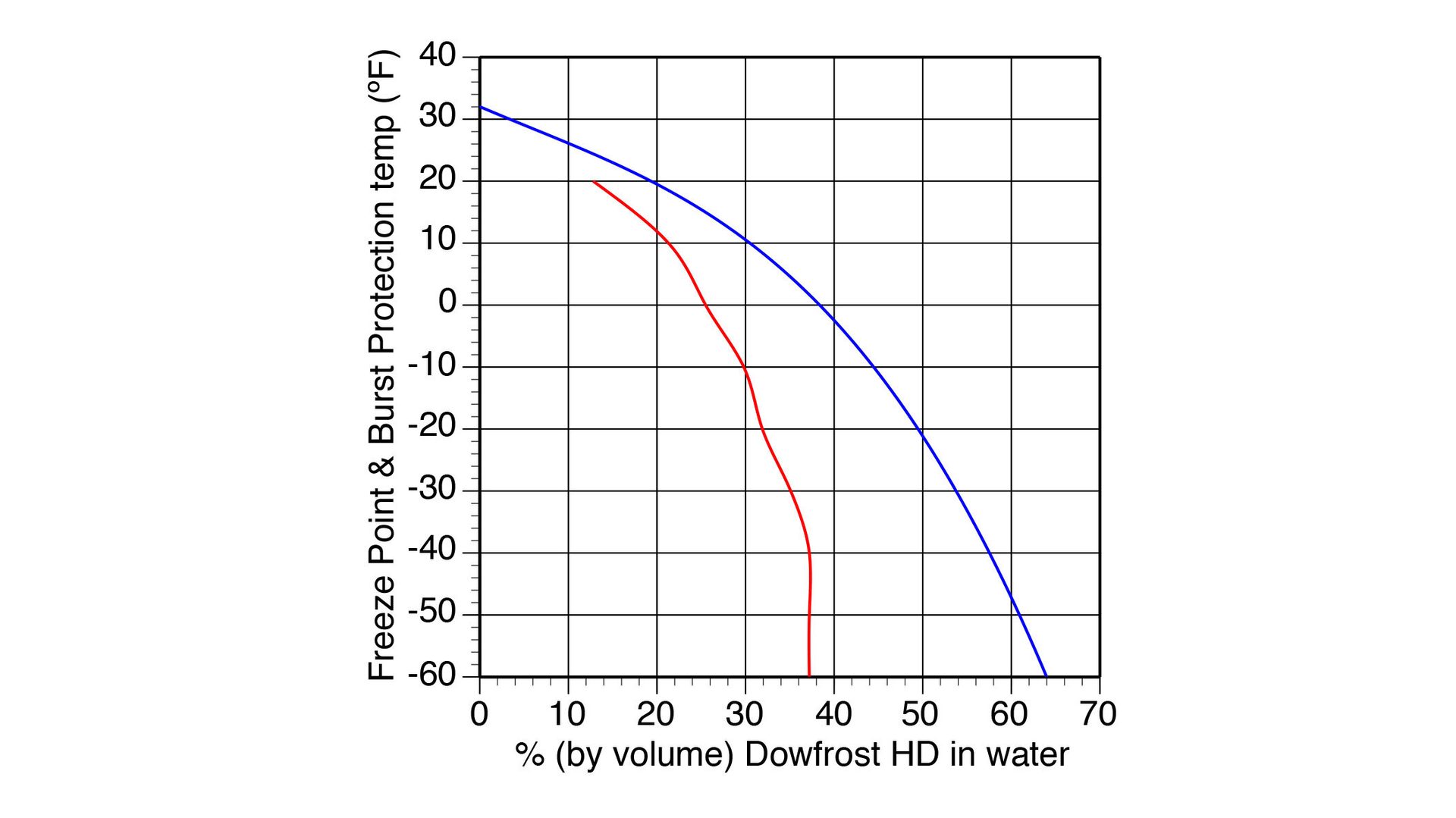
ENLARGE
FIGURE 8
It Works: Antifreeze solutions are the only way to protect unassisted hydronic systems from freezing. They are essential in geothermal earth loops, solar thermal systems and snowmelting systems. Most manufacturers of monobloc air-to-water heat pumps also require that antifreeze be used with their units. It protects them from freezing in winter, as well as against the formation of ice crystals in the heat pump’s evaporator during cooling mode operating under lower outdoor temperatures.
Adjust your designs to compensate for the differences in head loss and heat transfer capacity of antifreeze solutions. Upsize expansion tanks when necessary. Don’t use concentrations that are significantly higher than required. Check water quality against the specifications given by the antifreeze manufacturer. Periodically test the solution for both freeze point and inhibitor reserve levels. Finally, sleep well on the cold winter nights knowing that chemistry is at work to protect your systems.
Image courtesy of sturti / E+ / Getty Images
John Siegenthaler, P.E., is a consulting engineer and principal of Appropriate Designs in Holland Patent, New York. In partnership with HeatSpring, he has developed several online courses that provide in-depth, design-level training in modern hydronics systems, air-to-water heat pumps and biomass boiler systems. Additional information and resources for hydronic system design are available on Siegenthaler’s website, www.hydronicpros.com. Contact him at hydronicpros@gmail.com.